Empirical Formula of Ascorbic Acid
The whole-number ratio gives us the subscripts for the empirical formula. 545 16 340625 mol 340625 1 Now as you.
A compound composed of only carbon and hydrogen is 252 hydrogen by mass.

. What is the empirical formula of ascorbic acid given that the pseudoformula is C3407H453O3406. Determine the empirical formula of ascorbic acid if it is composed of 4092 carbon 458. Simplest or empirical formula.
The multiple is found by comparing the formula weight of the empirical formula with the molecular weight. Determine the empirical formula of ascorbic acid. Answer 1 of 3.
For example in Sample Exercise 312 we saw. Multiplier molar mass empirical weight 17612g empirical weight g empirical weight 3 x atomic wt. In 100 g of ascorbic acid we will have 4092 g C 458 g H and 5450 g O.
Empirical formula is the smallest whole number ratios of elements in a compound. Express your answer as a chemical formula. The whole-number ratio gives us the subscripts for the empirical formula.
Mol O the empirical formula is C3H4O32 Apr 2022 What is the empirical molar mass of ascorbic acid. The empirical formula of ascorbic acid can be described as the simplified form of the chemical formula of ascorbic acid where the ratio of each atom is still observed. QA By tamdoan January 19 2022 0 Comment what is the empirical.
What is the empirical formula of ascorbic acid given that the pseudoformula is C 341 H 453 O 341. What is the empirical of ascorbic acid. The empirical formula of ascorbic acid is C₃H₄O₃.
The dye has a percent composition of 7595 C 1772 N and 633 H by mass with a molar mass of about 240 gmol. Since nCnHnO is 3 mol C4 mol H3. C 4 x atomic wt H 3 x atomic weight O empirical weight.
A CHO B CH2O C C2H3O2 D C3H4O3 E none of the above. Show transcribed image text. Determine the molecular formula of the dye.
In 100 g of ascorbic acid we will have 4092 g C 458 g H and 5450 g O. Ascorbie acid vitamin C a white crystalline solid that is present in fruits and vegetables curves scury and may help. What is the empirical formula of ascorbic acid.
4092 12 341 mol 340625 1001 1 H. In this JC1 webinar lets learn how to calculate empirical and molecular formula of ascorbic acid or Vitamin CThe empirical formula of a compound shows the. Determine the empirical formula of ascorbic acid if it is composed of 4092 carbon 458 hydrogen and 5450 oxygen.
458 1 458 mol 340625 1345. Answer 1 of 3. Express answer as a chemical formula.
Given that the pseudoformula is c3407h453o3406.
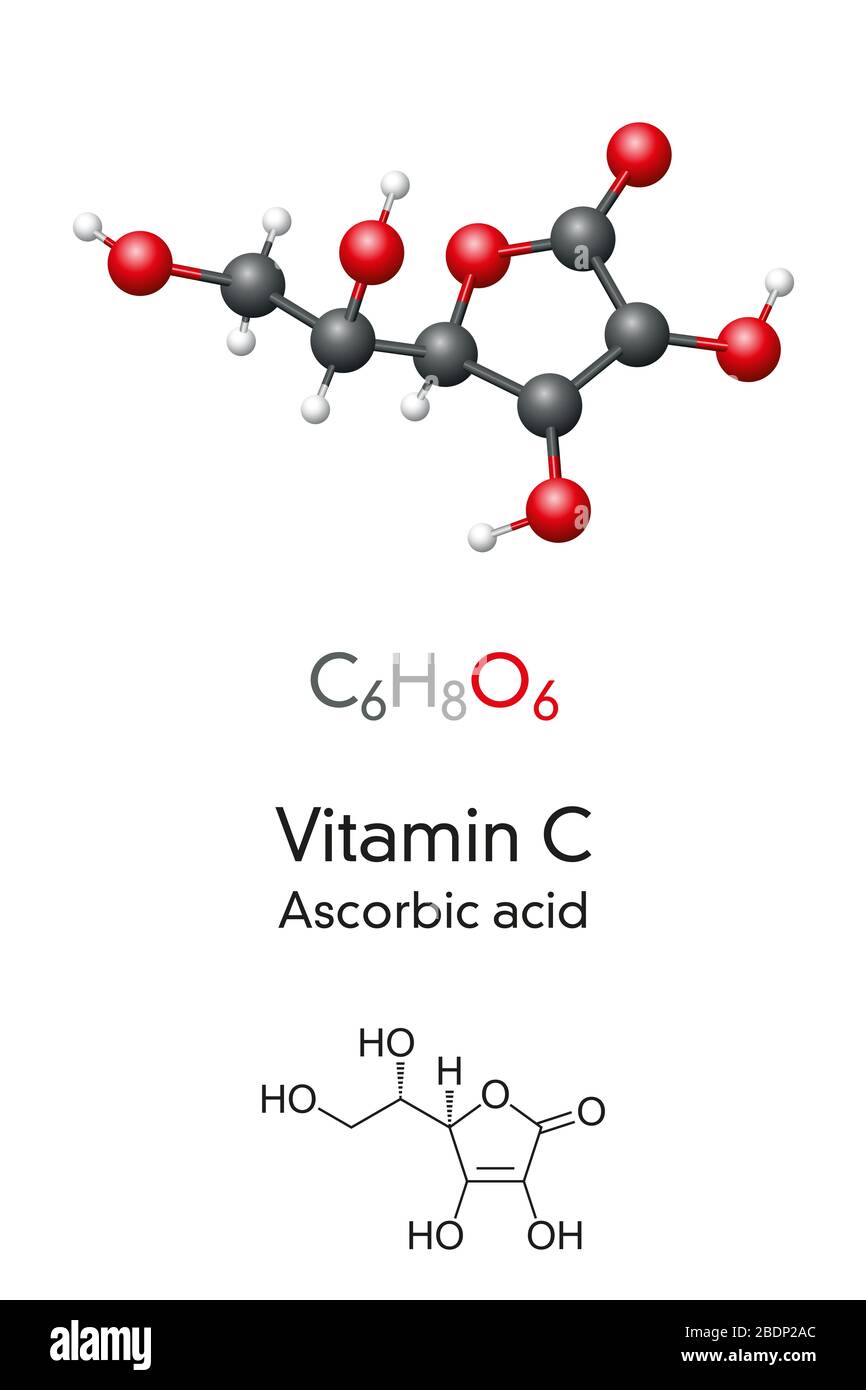
Vitamin C Molecule Model And Chemical Formula Ascorbic Acid Ascorbate Skeletal Formula And Molecular Structure Vitamin Found In Various Foods Stock Photo Alamy

Pin On Typography Design Handwritten
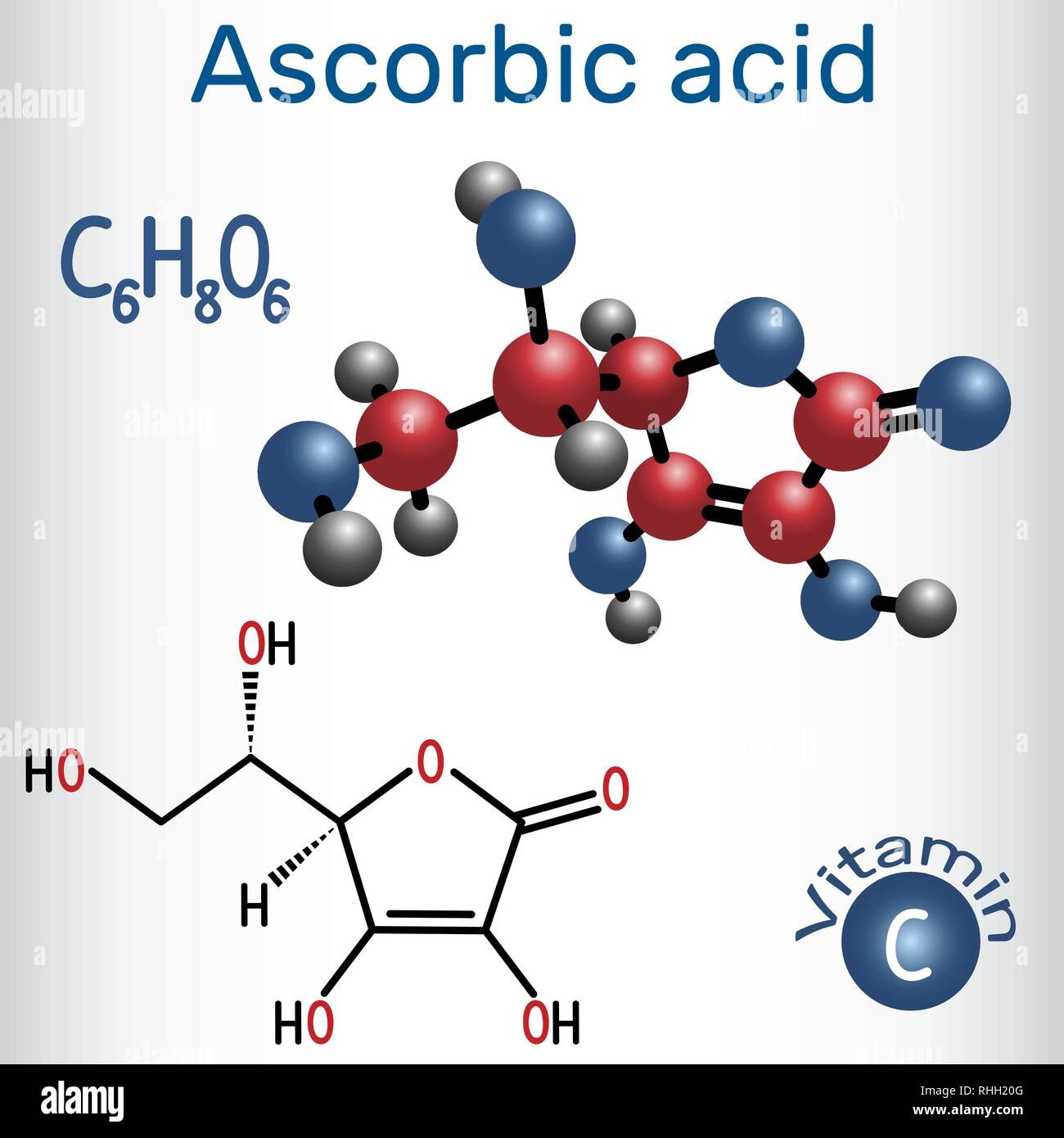
Ascorbic Acid Vitamin C Structural Chemical Formula And Molecule Model Vector Illustration Stock Vector Image Art Alamy
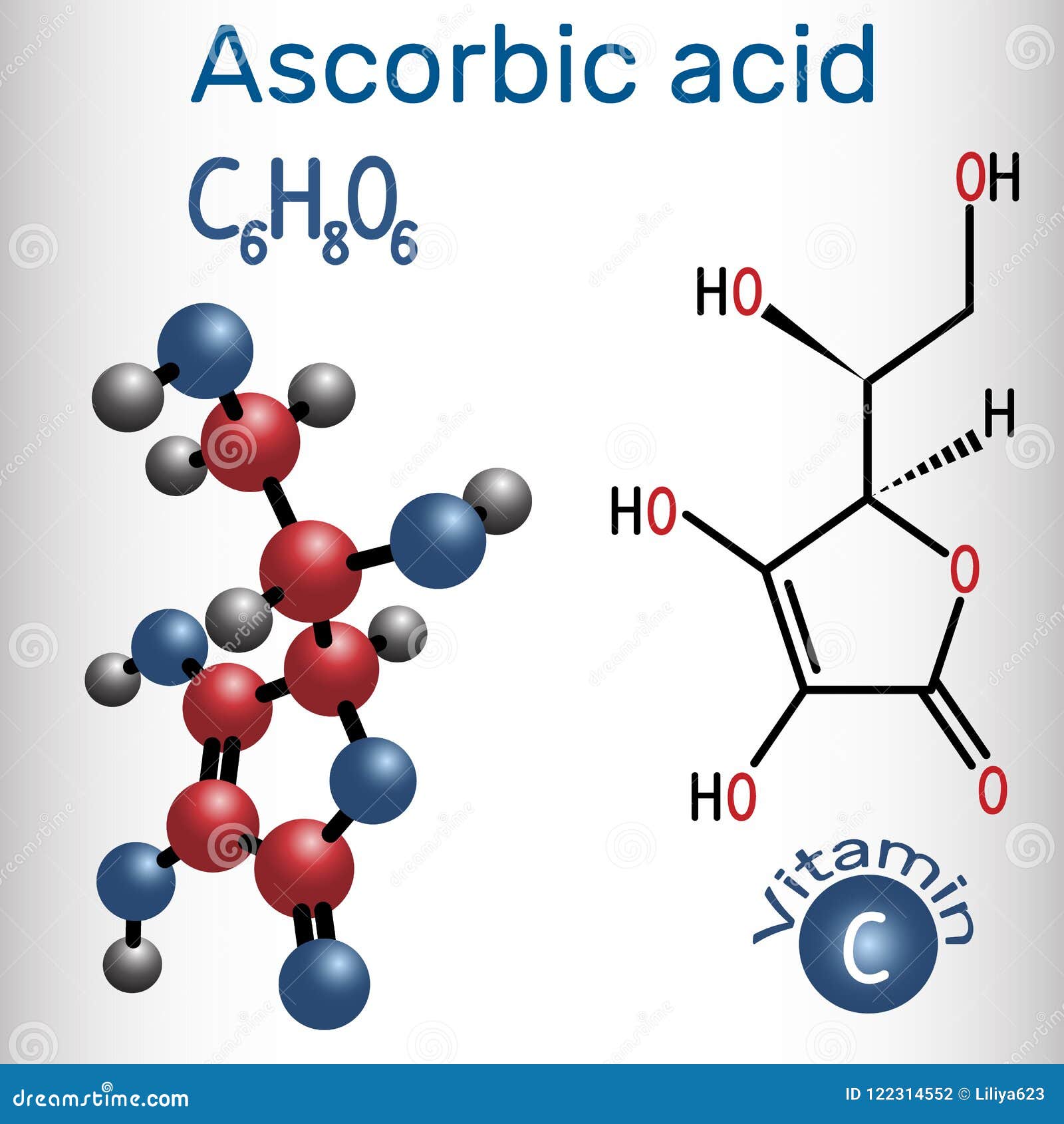
Ascorbic Acid Vitamin C Structural Chemical Formula And Molec Stock Vector Illustration Of Radical Antioxidant 122314552

No comments for "Empirical Formula of Ascorbic Acid"
Post a Comment